BDD: Innovating Drug Delivery and Clinical Research
BDD was founded as Bio-Images Research Ltd in 2000. Initially its expertise was focused on early phase clinical research for the pharmaceutical industry using gamma scintigraphic imaging. These studies provide visualizations of dosage forms inside humans, and therefore an understanding of how pharmaceutical products work, which is incredibly useful in product development within the industry. This research remains today as one of the business’ two specialty divisions.
In 2010, the enterprise founded its sister company and second specialty, Drug Delivery International Ltd, to deliver its patented OralogiK™ technology and formulation development services. These two entities came together in 2017, and merged into BDD Pharma Ltd (BDD). The company has since developed an integrated clinical and manufacturing service that allows for the rapid evaluation of products in humans, saving its customers a great deal of time and money during product development.

The company’s range of services cater to the individual needs of each client, whether they are troubleshooting, developing a new formulation or testing a product in vitro (in the lab) or in vivo (inside a human). In doing so, BDD calls upon its imaging and pharmacokinetic techniques, as well as its knowledge of biopharmaceutics. A key area of the organization’s focus is the development of unique, targeted release profiles with oral drug delivery systems. BDD is an expert in utilizing hot melt extrusion, wet and dry granulation and different coatings in the development of innovative drug products. Its partners are spread all over the world and range from small virtual biotechs to large global pharma companies.
Treatment solutions
In discussing BDD’s patented release technology, Carol Thomson, Chief Executive Officer, states: “We developed OralogiK™ as an attractive alternative to diffusion-based matrix release or coated multiparticulate technologies for controlled release. OralogiK™ is neither pH dependent nor reliant on uniform conditions within the Gastrointestinal Tract (GIT), whereas issues in the GIT system relating to stasis, variable fluid volumes or motility can have negative consequences for diffusional drug release mechanisms. Our solution is uniquely based on an erosion technology that is not impacted by these factors and this allows us to create complex release profiles that are otherwise unachievable.
“This erodible layer is compressed around a tablet containing the Active Pharmaceutical Ingredient (API), which provides the specific dose. This layer is designed to break down at a consistent rate, independent of its location within the GIT, allowing us to create lag times of up to 12 hours between the dose being taken and the drug entering the patient’s system. Its real USP is the way in which it can operate regardless of biological factors such as pH changes, agitation and stomach contents, and doesn’t rely on the patient’s gut microbiome or enzymatic composition.
“This technology has been repeatedly tested in the clinical setting and can be tailored specifically to our partners’ requirements. In an alternative sustained release configuration, an API can be incorporated into the external later as well, allowing for earlier absorption once the erosion process has begun.” The OralogiK™ mechanism has also been applied to a colon targeted product, CologiK™, which aims for a more proximal release in the lower regions of the GIT.
OralogiK™ is also playing a pivotal role in an exciting project, as Carol explains: “We are delighted to be working so closely with our partner at Contera Pharma. What they are doing in the space of neurology and rare disease, including Parkinson’s disease, is really exciting.
“During the course of Parkinson’s disease (PD), mobility problems and recurring tremor early in the morning, before the first intake of levodopa, are part of the development of motor complications associated with sustained oral levodopa treatment of PD. Recent surveys have indicated that early morning akinesia (or morning OFF episodes) are present in around 60 percent of PD patients across all disease stages and that a similar number of patients are affected by nocturnal immobility with difficulties turning in bed. As such, nocturnal immobility and morning akinesia are key medical unmet needs and significant contributors to impaired sleep quality and reduced quality of life for patients. There are
currently no effective, reliable, and easy to use treatment options available to satisfactorily address these problems.
“The partnership with Contera Pharma is focusing on the development of a novel tablet formulation of levodopa / carbidopa (CP-012) to specifically treat PD patients suffering from nocturnal or early morning motor complications. The product we are collaborating with Contera on aims to reduce these complications utilizing our OralogiK™ formulation technology, and we expect to be able to test this novel principle in a Phase I clinical study in healthy volunteers this year ahead of continuing the development in Parkinson’s disease patients.”
Scientific and service excellence
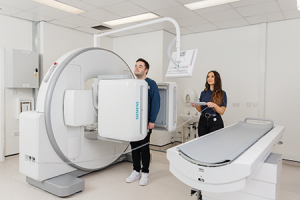
For over 20 years, BDD has been utilizing gamma scintigraphy to give its clients a greater understanding of how their formulations perform in vivo. To facilitate further studies into dosage forms such as tablets, capsules, respiratory formulations and devices, drinks, eye drops and nasal administrations, the company has recently invested in a new, state-of-the-art camera. As Carol details: “Our new Siemens Healthineers Sybia Evo Excel gamma camera features dual-head imaging, which will allow us to increase both the capacity and efficiency of our scintigraphy studies. As a world leader in our field, this investment will help us maintain our position of hosting the most cutting-edge technologies and abilities.
“At BDD, our ethos and commercial drive hasn’t wavered from our founding principles,” she concludes. “We are growing at a rapid rate, so a lot of our current focus is on maintaining our levels of both scientific and service excellence, as well as working with our partners to create and develop life-changing medications and treatments. The more we grow, the more we are able to bring new and exciting patient-focused products to the market.”
