Bioquell
Reducing risk
A global pioneer in biodecontamination systems and services, Bioquell focuses on protecting its customers’ work from biological contaminants through the delivery of innovative, industry-leading solutions
Hampshire-based business Bioquell has been providing solutions to decontaminate facilities since 2000, yet its history can be traced back to 1730 when William Dent and Lancelot Burton 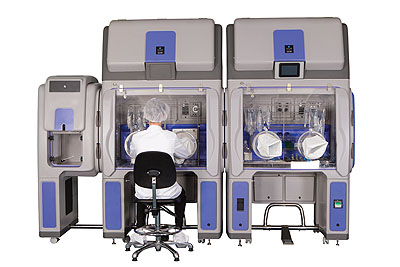 founded a sanitation business in London. In the many years that followed, said business branched out into steam sterilisers and manufacturing solutions such as autoclaves for hospitals to sterilise surgical instruments, and later into contamination and environmental health control technologies, including the supplying of specialised filtration and air conditioning products to the defence sector.
founded a sanitation business in London. In the many years that followed, said business branched out into steam sterilisers and manufacturing solutions such as autoclaves for hospitals to sterilise surgical instruments, and later into contamination and environmental health control technologies, including the supplying of specialised filtration and air conditioning products to the defence sector.
In 2000, the company, at the time known as MDH, was still involved in infection control, albeit utilising different technology and with a much larger geographical footprint, with businesses located in the United States, China, Singapore, France and Ireland. “To reflect the strides the company had made, it was decided that it would change its name to Bioquell, with MDH continuing to serve the defence sector,” explains Ian Johnson, Bioquell’s Executive Chairman. “Today, we possess world leading expertise in hydrogen peroxide vapour bio-decontamination technology which has been shown in independent studies to provide the highest efficacy in controlling biological contamination in pharmaceutical, life sciences and hospital environments.”
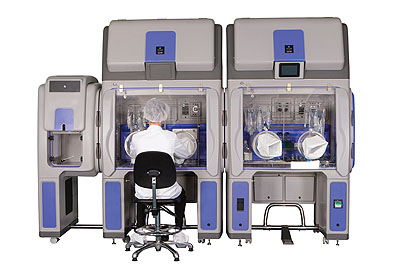
Bioquell offers a range of equipment for customers to purchase depending on the application, as well as a rapid bio decontamination service (RBDS) where its trained engineers carry out the work for its customers. “The introduction of our ‘Vision, Mission and Values’ has enabled us to mould and develop our business and our team,” Ian continues. “Our strength is undoubtedly our people, who are responsible for driving our culture and enabling our focus to be directed towards customer centricity, innovation, integrity, quality, safety and trust.”
While approximately 80 per cent of the company’s revenue is now generated by selling products and services outside of the UK, its facility in Andover is the sole design and manufacturing site for all of its products. “It is predominantly an assembly facility, with our designed parts sourced mostly from within the UK, and our main depth of manufacturing is our state-of-the-art in-house rotomoulding facility, delivering complex and technically challenging parts for all of our products,” Ian details. “We moved into this facility in 2008, and all of our product lines have been designed with growth in mind. It is currently running at around 60 per cent capacity and as we grow, our ISO 9001:2015 standard enables us to utilise and expand our workforce and train new operators as the lines require full manning or second shifts. We take pride in our ability to flex our skilled employees onto multiple products, whilst still maintaining training and quality standards.”
Upgrading to ISO 9001:2015 with zero major nonconformities has been one of the company’s greatest achievements, and has helped to ensure its customers receive an exceptional level of standard throughout its entire end-to-end process. Meanwhile, investing heavily in its programme of New Product Introduction (NPI) has enabled Bioquell to develop innovative new products utilising lean manufacturing best practices and the best technology.
“Lean has been at the forefront of our continuous improvement process for the past five years,” adds Rob King, Bioquell’s Manufacturing and Quality Director. “We have provided lean-based training to multiple staff members from all departments, and we continue to carry out improvement projects and Kaizen events. Not only has this improved our foundations for delivering world class manufacturing, but in 2017 we were able to take £190,000 worth of costs out of the business, and we are currently on target to further reduce product cost by ten per cent in 2018.”
There is certainly no shortage of case studies that one can refer to when looking at the work carried out by Bioquell. One example of note involved the newly constructed Sidra Medicine hospital in Doha, Qatar, and its requirement for a high level of disinfection of key clinical areas in a short timeframe. Bioquell was specifically chosen to perform a 6-log decontamination of identified areas consisting of 450 rooms totalling 56,000 cubic metres, including a suite of 12 operating theatres, sterile processing units and wards for high risk patients. By working alongside numerous departments at Sidra Medicine, the project was successfully completed on scheduled within a 15-day timescale. Since its successful delivery, Sidra Medicine has purchased a number of suites of Bioquell equipment to perform regular decontamination cycles of single rooms and other small areas within the hospital.
Successful projects like that which is detailed above have helped to contribute to the company being shortlisted in the highly competitive Best Healthcare & Pharmaceutical PLC category of the 2018 UK Stock Market awards. Now in their eighth year, the awards are considered as being one the leading ceremonies recognising UK publically listed companies and are compiled by senior individuals from the world of corporate finance, law, financial services, accounting, public relations and the media.
Moving forward, Bioquell’s focus will be on its continuing efforts to build a world class business committed to delivering risk reduction solutions to its many customers, and Ian knows what will be the key ingredient in achieving this aim. “Bioquell would not be where it is today without its dedicated, hard working team,” he concludes. “We are excited to have young blood working its way through the ranks, supported by our many long-standing members of staff that have been with us for a number of years. It is they who continue to share guidance, advice and experience with our younger team members who will themselves be instrumental in helping us to succeed in the years to come.”
Bioquell
Products: Biodecontamination systems