SiO2 Materials Science
The science behind success
Utilizing advanced, patented materials science, SiO2 develops and manufactures medical grade containers with a glass-like barrier, which meet the most demanding of needs of next generation biological drugs and molecular diagnostics
SiO2 Materials Science (SiO2), is an advanced materials sciences company which has been spun out of a 110-year-old family business focused on flexible, advanced, manufacturing. SiO2 has spent 10 years and $500 million in research & development to invent a breakthrough technology platform, which has a number of industrial applications. Through this platform, the 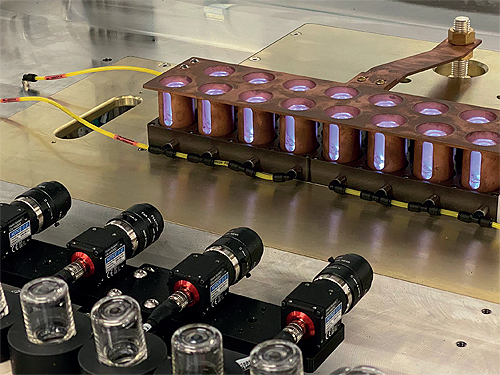 company has invented a new hybrid material that fuses the benefits of glass and plastic without the drawbacks of either. Currently, the company is focused on two segments: Pharmaceuticals and Molecular Diagnostics.
company has invented a new hybrid material that fuses the benefits of glass and plastic without the drawbacks of either. Currently, the company is focused on two segments: Pharmaceuticals and Molecular Diagnostics.
The SiO2 technology platform coupled with a 50-year track record of flexible manufacturing, strategically positions the company to capture a majority share of the primary packaging for the growing biological drug and genomic testing markets. The platform uses a modified plasma enhanced chemical vapor deposition (PEVCD) process to apply multiple layers of a barrier coating to any polymer / plastic substrate. The layers combined to be less than 20 nanometers – or 50 times thinner than a human hair – and provide a highly effective barrier to environmental gases and leachates.
Working with a host of large academic research centers, including those located at the Massachusetts Institute of Technology, University of Chicago, Harvard University, the UC Santa Barbara, and Auburn University – close to which sits SiO2’s manufacturing facilities – the company now boasts over 350 patents and more than 8000 patent use claims.
“Over the last ten years or so, we have invested upwards of half-a-billion dollars developing the science needed to fuse glass and plastic,” states Lawrence Ganti, SiO2’s President of Customer Operations and Chief Business Officer. “What our efforts have yielded is a technology platform which facilitated the creation of a new material, one which takes an exterior medical grade polymer or plastic shell, and applies a nanolayer of pure silicon dioxide on the inside using PECVD. While this is not a new technology as such, having been used in the microchip industry for a number of years previously, this does mark the first time it has ever been modified for medical purposes. We materialized that technology into two categories, one being pharmaceuticals and biotech, and the other molecular diagnostics, and SiO2’s technology is now used by host of customers in each of the above segments.”
Innovative products
The secret behind SiO2’s success as a business is clearly rooted in the science behind its technological platform, but also its historical track record of flexible manufacturing. The two have been used to answer specific modern challenges presented by the use today of standard glass or plastic. “For more than a century, people have been using glass as a means of packaging sensitive materials, whether it be drugs, human blood or other specimens. However, in all that time, there has been very little in the way of innovation,” Lawrence explains. “In recent years, however, there have been several big factors that have facilitated the need for new ideas. One has been the advances made in automation, specifically the use of automated manufacturing lines. While this has sped up the process of filling glass containers, it also presents a breakage issue, where having broken vials or jars can result in the shut- down of entire lines. Such instances have driven a need for change, particularly when it comes to the durability of vials etc.
“The other significant development has been the fact that the properties of the drugs being packaged and the sensitivity levels associated with diagnostics have advanced tremendously. On the drugs side, we have seen a big increase in the use of complex biological medicines, which tend to be super sensitive to non-inert contaminants, which can come in the form of things like glass particles or oil within in a glass chamber. Diagnostics wise, the use of super sensitive imaging technology to scan bloods for DNA means there is a need to avoid any and all impurities that may appear within a particular container.
“These factors have resulted in a pressing demand for a material that is not only strong enough to offset the risk of breakage, but is also 100 per cent inert. At first, few thought this to be possible, but with our technology we have been able to create a product that possesses the best qualities of both glass and plastic, without the negatives of either, and this truly sets SiO2 apart from anyone else operating within our field.”
Major funding boost
Today, SiO2 finds itself tasked with undoubtedly its most important mission to date, having been selected to form part of the U.S. Government’s Operation Warp Speed program to support the development and supply of Covid-19 vaccines and therapeutics. For its part, the company has received a $143 million contract to accelerate capacity scale-up of its advanced primary packaging platform for storing said future medications. “This investment will be used to scale-up our manufacturing capacity multiple times over, taking it from around ten million vials per year to approximately 140 million, and all in the space of just a few months,” Lawrence confirms.
Prior to said investment, SiO2’s activities centered solely on its 165,000-square foot manufacturing facility in Auburn. In addition to using some of the capital to restructure half of that building to install new clean rooms, the company has also purchased several new units in the surrounding area. These include two 65,000-square foot buildings that will be refitted with medical grade cleans rooms and additional equipment to support an increase in manufacturing capacity, another 40,000-square foot unit that is currently being green fielded, and a fifth facility that is in the process of being constructed.
Sense of purpose
Having been granted a Defense Priorities & Allocations System (DPAS) rating by the U.S. Government, SiO2 has been given priority status for construction resources, which has allowed it to overcome the challenge of bringing contractors onboard at relatively short notice to carry out its expansion. The company will also be looking to eventually add as many as 300 new employees to support its growth, and the positive publicity that it has received from being selected to be part of Operation Warp Speed has helped in attracting strong talent into the business.
One of Lawrence’s most important tasks in recent months has been to assist both this new talent and the company’s existing staff in embracing a change in culture as it has transitioned from being a strictly R&D focused business into one that now also possesses an important commercial element. “Anytime you want to implement change, the best time to do so is when there is a common cause or message that everyone associated with the business can understand and get onboard with,” he says. “At SiO2, that message is clear, and that is that with every new vial we ultimately help to produce, that vial is contributing to saving someone’s life. Collectively, we realize that Covid-19 is a disease that affects everyone on our planet, and the response from our people to the need to up-scale rapidly has been nothing short of incredible. The sense of purpose that exists within the business today is phenomenal, and there is a real sense that nothing is impossible when it comes to what we can do to assist in the fight against Covid-19.”
Nimble business
Prior to the onset of the Covid-19 pandemic, SiO2 had no less than 16 projects ongoing with different companies to develop containers for their respective biological drugs, and this work has continued relatively uninterrupted, which is again testament to its people. Meanwhile, SiO2’s involvement in Operation Warp Speed – and the subsequent investment that this has brought – has not only aided in the scale-up of the business, but also allowed it to bring in additional resources and improved processes, effectively accelerating its businesses plans by some three-to-five years.
“If the last six-months or so have taught us anything, it has been of the importance of being nimble as a business,” Lawrence goes on to detail. “We have had to pivot SiO2 significantly in this time to ensure that our processes and products are perfectly positioned to support the development of the latest biotech drugs and the new generation of vaccines that will be heading our way in the years to come. This ability is likely to become all the more important in the future. For example, today our customers are predominantly working with vials for drugs, however it is likely that in the next 12 months there will be a shift towards syringes, and we need to be ready for that if we are to remain relevant to their needs. Building continued flexibility into our processes and manufacturing activities will continue to be key to our success, and will help us to redefine what it means to be nimble.
“As for what the future holds, I definitely see SiO2 continuing to grow at a consistent rate and supporting our customers by providing the flexible manufacturing services that they need. That service – combined with our willingness to take the time to listen to said customers in order to fully understand their needs – is what we really excel at, and is what we believe will go a long way to contributing to both our customers and SiO2 achieving their respective long-term goals.”
SiO2 Materials Science
Products: Advanced Primary Packaging Platform
www.sio2ms.com
