Talley Group
Reducing avoidable harms
Talley Group excels in the production of Class IIa medical devices, differentiating itself through unparalleled technical capabilities
More than 60 years ago, the pioneering vision of Talley Group’s founder (Henry Talley) inspired the opening of a whole new marketplace in the UK. It was indeed the Romsey-based company that first introduced pressure relieving mattresses to the country, later becoming the first business to rent these products, too. Still family-owned to this day, the designer and manufacturer remains a market leader by continuously increasing its production capabilities and advocating the need for higher quality standards to be established and maintained across the industry.
“A huge strength of the company is that it is both horizontally and vertically-integrated,” begins Talley’s Operations Director, Kevin Mearns. “We are capable of performing every single 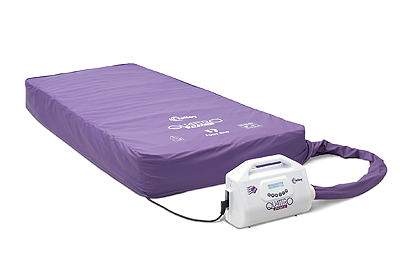 production-related function from our site, here in Romsey, which gives us full control over all the products that we design and manufacture. As a result, we have been able to create a very diverse portfolio and we export our products to some 40 countries worldwide.”
production-related function from our site, here in Romsey, which gives us full control over all the products that we design and manufacture. As a result, we have been able to create a very diverse portfolio and we export our products to some 40 countries worldwide.”
It is a source of great pride for Talley that the company uses only UK-manufactured components. “Unlike many of our competitors who import ready-made parts from abroad, we do not. We take in raw materials and then conceptualise, design, prototype, and build a mattress,” Kevin clarifies. “Another key advantage of ours is that all of our products are designed with a very specific purpose – either to reduce pressure ulcer incidence, treat acute and chronic wounds or to apply intermittent pneumatic compression. They are not made with the idea of being used in other sectors, which allows us to focus entirely on the requirements of the healthcare market and to keep a very close check on what we have to do, in order to stay relevant and efficient.”
Such a line of reasoning is demonstrated in Talley’s investment activities, when it comes to the company adding to its equipment. Kevin explains: “We have an ongoing investment programme that aims to keep us ahead of the game and ensure that we are quick to adopt any new materials or processes that become available in manufacturing. For example, five years ago, we did not have 3D printers, now we have two. We did not have computer-controlled five-axis milling machines, now we operate a few of those. All in all, we are skilled in every process that is needed to make our products.”
For it to maintain these high levels of craftsmanship, Talley is also running an apprenticeship programme where the company teaches aspiring youngsters the sophisticated engineering and manufacturing processes that are involved in the creation of a Talley-branded mattress system. “Bringing young people with fresh ideas gives impetus to the department they join. What is more, having an apprenticeship scheme helps to tackle the skills shortage crisis in the UK manufacturing industry by keeping and developing specific capabilities locally,” Kevin observes.
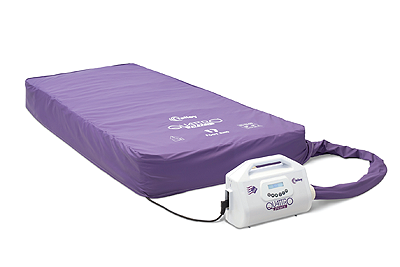
For many years, the Quattro range of mattresses has been synonymous with Talley. Sales Director, John McEwen, joins the conversation to discuss the company’s core product: “The two most popular systems in the range are the Acute and the Plus. The former offers a unique DEEP CELL THERAPY technology that allows the cells of the mattress to run at lower internal pressures. This minimises the pressure applied to the patient’s skin and subcutaneous tissues during the cycle. As for the Plus product, it is designed to reduce pressure ulcer incidence. What is impressive about these systems, is that they feature an active 1-in-4 cell cycle providing more support and comfort for the patients, whereas the majority of our competitors’ products are 1-in-2.
“Moving on, we also provide a range of negative pressure wound therapy products – the Venturi, which has been in the marketplace for over ten years now,” John adds. “In addition, we manufacture intermittent pneumatic compression systems and in both these areas, we compete successfully against significantly bigger players on the global stage.”
With its end users often being elderly and/or immobile people, it is essential for the company that only tried-and-tested and robust designs find a place in the market. Kevin comments: “We  are very keen to highlight the changes that are being initiated within the medical device regulations area. We are one of the very few manufacturers globally that can manufacture Class IIa medical devices. Unfortunately, a lot of other companies do not have the same technical capability, but they still tend to put their products in the market claiming that they meet higher standards. To us, it is unacceptable to not be able to prove that your product has a very detailed technical file and a complete and utter regulatory approval. More importantly, all of your processes need to be scrutinised by an external body. We have a notified body coming in to make sure that everything we say we do, is true and in compliance with regulations.
are very keen to highlight the changes that are being initiated within the medical device regulations area. We are one of the very few manufacturers globally that can manufacture Class IIa medical devices. Unfortunately, a lot of other companies do not have the same technical capability, but they still tend to put their products in the market claiming that they meet higher standards. To us, it is unacceptable to not be able to prove that your product has a very detailed technical file and a complete and utter regulatory approval. More importantly, all of your processes need to be scrutinised by an external body. We have a notified body coming in to make sure that everything we say we do, is true and in compliance with regulations.
“In my opinion, extra pressure from the industry, the regulatory bodies, and the clinicians themselves is required to make transparency in how the products are made an important requirement,” Kevin suggests. “There has been some progress as clinicians are well aware of the issue, but there is still an enormous amount of work to be done. For our part, we will continue to bang the drum and insist on the close assessment of the properties of every product that claims to be treating the vulnerable people we are trying to help.”
Serving a number of markets worldwide, the last few years have seen Talley achieve a steady growth in countries such as Belgium, Finland, the Netherlands, Ireland, and Australia. Moreover, the company has made great strides in Italy and Singapore as of late with its negative pressure range. “Because the UK is already quite a mature market, our growth here is driven a lot by the rental side of the business and the complementary services that we offer,” John remarks.
Having set up a long-standing presence across Europe, Talley is now setting its sights on growing on the other side of the Atlantic. “Clearly, the US market is very different from the one in the UK and most other European countries,” Kevin points out. “As it operates under the Medicare and Medicaid systems of reimbursement, the majority of operators are driven purely by cost, so one of the challenges for us is to be able to offer a cost-effective solution that fits in with the reimbursement requirements. We then have the logistical difficulties that inevitably arise when trying to manage such an enormous market. If one distributor per country does the job in Europe, this is not the case in the US where we will probably need more partners. Finally, we should remember that a lot of our products are rented, meaning that we need to have someone in place to collect them once the rent is over, decontaminate them to fairly stringent regulations, and make them available for the next client. At the moment, John is heavily focused on working around these challenges and finding the best methods of servicing the US.
“It is the demographics of our clientele that determines our expansion within certain marketplaces,” Kevin goes on. “As medical science progresses, life expectancy increases and one of the unfortunate consequences of that is the fact that a larger number of people would suffer from pressure damage, because they have to spend more time in bed. You also have to factor in the development of conditions such as dementia that really debilitate patients and make them immobile for long periods of time. Therefore, we continuously look how we can drive innovation and incorporate the latest technology in developing products that alleviate the problems faced by this particular demographic.”
Boosting its salesforce further, both in the UK and overseas, is a key short-term objective for Talley, as the organisation is eager to address the issue of changing demographics, ensuring that its offer is clinically and financially solid. “Looking three-to-five years ahead, the plan for the group is to grow in double digits year-on-year. To this end, we intend on releasing new products and investing in our manufacturing processes to make sure that we maintain the quality we have become renowned for,” John concludes.
Talley Group
Products: Pressure relieving mattresses; negative pressure wound therapy products; intermittent pneumatic compression system